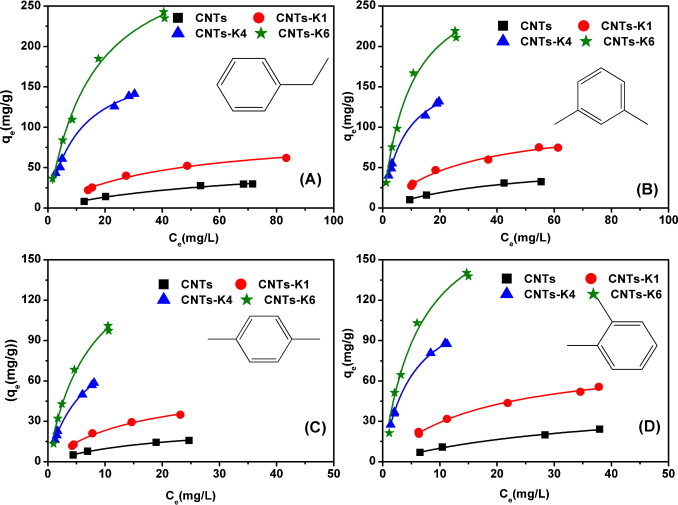
ABSTRACT: To investigate the effect of pore structure and surface chemistry on adsorption of monoaromatics, multi-walled carbon nanotubes (MWCNTs) with different specific surface area (SSA) and surface oxygen content by KOH activation treatment have been prepared. The results indicate that adsorption of four test chemicals is enhanced by 2–3, 7–9 and 10–13 times for CNTs-K1 (MWCNTs:KOH
=
1:1), CNTs-K4 (MWCNTs:KOH
=
1:4) and CNTs-K6 (MWCNTs:KOH
=
1:6) as justified by the ratios of adsorption coefficient. Adsorption properties of ethylbenzene and xylene isomers (EX) by activated-MWCNTs depend significantly on mesopore volumes and hydroxyl groups. There are strong positive correlations between the adsorption capacity (or adsorption affinity) and mesopore volumes or hydroxyl groups. Mesopore-filling could be a predominant mechanism governing monoarmatics adsorption onto activated-MWCNTs, contributing more than simply increasing SSA. The hydroxyl groups at low concentration can improve diffusion of MWCNTs in water, which could be favorable for aqueous phase adsorption. However, it is not large enough to form a lot of water clusters to hinder EX adsorption. This is the first systematic study of the influence of physicochemical properties on adsorption of monoaromatics onto activated-MWCNTs by qualitative and quantitative analysis, and results highlight the synergetic effects of porosity and surface chemistry in controlling adsorption of monoaromatics onto MWCNTs in wastewater treatment.