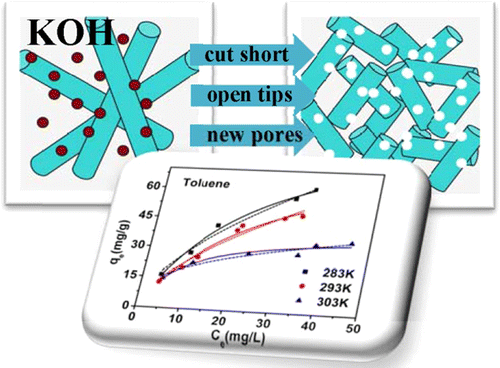
ABSTRACT: Multiwalled carbon nanotubes activated by KOH (CNTs-KOH) were synthesized and employed as adsorbents to study adsorption characteristics of toluene, ethylbenzene, and m-xylene (TEX) from aqueous solutions. Kinetics data were fitted by pseudo-second-order model, and intraparticle diffusion was not the only rate-controlling step. Adsorption isotherm data could fit well with Langmuir, Freundlich, and Dubinin–Radushkevich (D-R) models. The maximum adsorption capacities on CNTs-KOH are 87.12, 322.05, and 247.83 mg/g for toluene, ethylbenzene, and m-xylene, respectively. The adsorption capacities of TEX onto CNTs-KOH increased with contact time and decreased with temperature and are not significantly affected by humic acid. However, Cr6+ could decrease adsorption of TEX by 17.66, 4.51, and 12.69% as (Kd1 – Kd2)/Kd1, respectively. The thermodynamics parameters indicated that adsorption was a feasible, exothermic, and spontaneous process in nature and a physisorption process. The present CNTs-KOH show a better EX adsorption performance than other adsorbents, suggesting that CNTs-KOH are promising EX adsorbents in wastewater treatment.