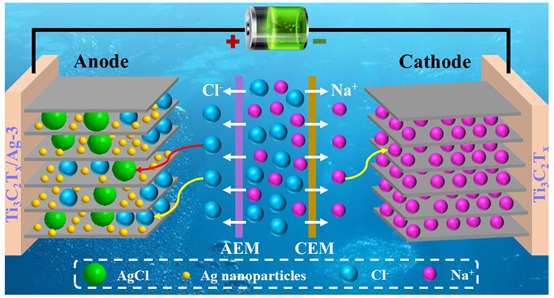
Abstract: The recent advances in chloride-ion capturing electrodes for capacitive deionization (CDI) are limited by the capacity, rate, and stability of desalination. This work introduces Ti3C2Tx/Ag synthesized via a facile oxidation-reduction method and then uses it as an anode for chloride-ion capture in CDI. Silver nanoparticles are formed successfully and uniformly distributed with the layered-structure of Ti3C2Tx. All Ti3C2Tx/Ag samples are hydrophilic, which is beneficial for water desalination. Ti3C2Tx/Ag samples with a low charge transfer resistance exhibit both pseudocapacitive and battery behaviors. Herein, the Ti3C2Tx/Ag electrode with a reaction time of 3 h exhibits excellent desalination performance with a capacity of 135 mg Cl- g-1 the 20 mA g-1 in a 10 × 10-3 m NaCl solution. Furthermore, low energy consumption of 0.42 kWh kg-1 Cl- and a desalination rate of 1.5 mg Cl- g-1 min-1 at 50 mA g-1 is achieved. The Ti3C2Tx/Ag system exhibits fast rate capability, high desalination capacity, low energy consumption, and excellent cyclability, which can be ascribed to the synergistic effect between the battery and pseudocapacitive behaviors of the Ti3C2Tx/Ag hybrid material. This work provides fundamental insight into the coupling of battery and pseudocapacitive behaviors during Cl- capture for lectrochemical desalination.
Keywords: battery behavior, capacitive deionization, chloride-ion capturing, pseudocapacitive behavior, Ti3C2Tx/Ag