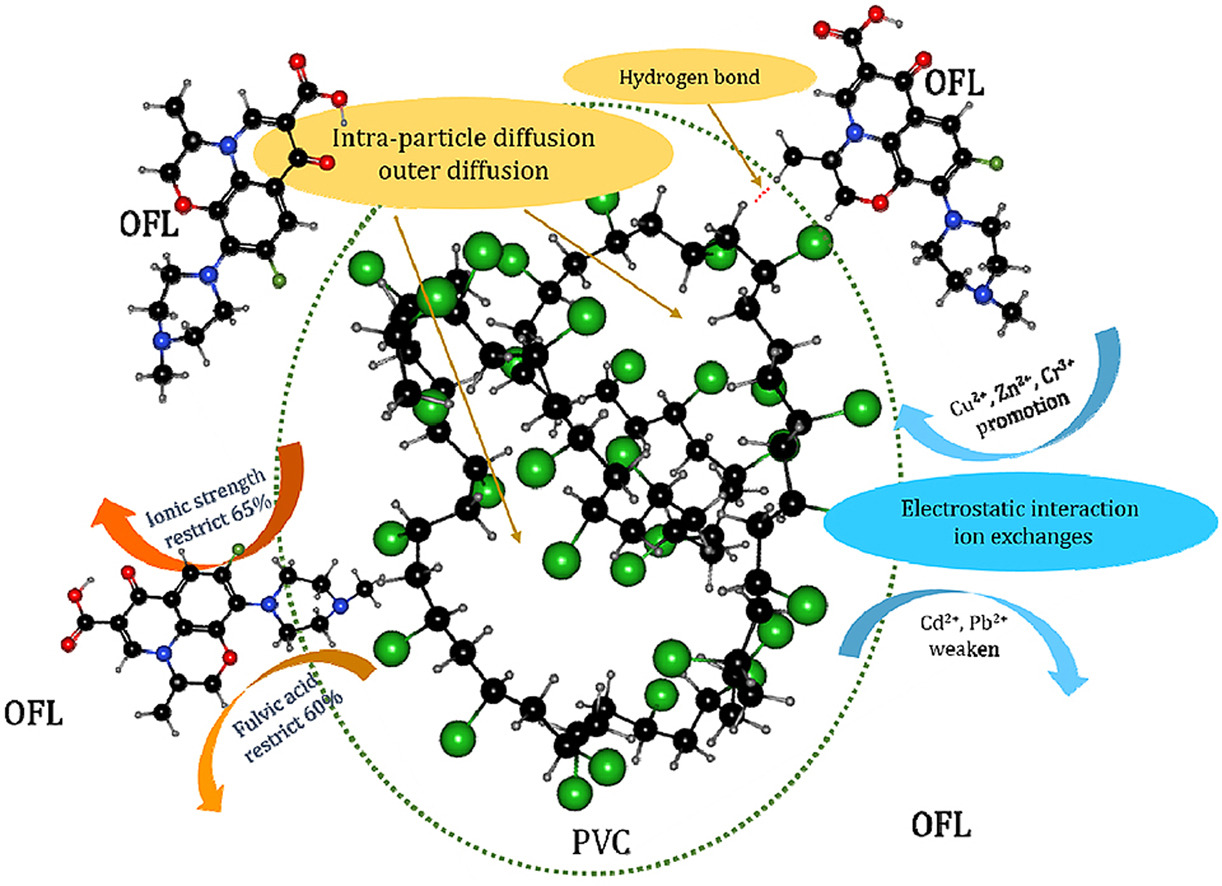
Abstract: In recent years, the composite pollution of microplastics with organic pollutants and heavy metal ions in the water environment, including their combined toxicity, has received increasing attention. However, the mechanism underlying the joint effect of antibiotics and heavy metals on the surface behavior of microplastics has not been reported. The primary purpose of this article was to analyze the adsorption of levofloxacin (OFL) onto polyvinyl chloride (PVC) in an aqueous solution. The adsorption behavior was studied using kinetics, thermodynamics, and isotherm models, and the effects of several environmental factors, such as ionic strength, fulvic acid, and heavy metals, were determined. The adsorption kinetics and isotherms models indicated that the whole adsorption process was controlled by both intraparticle and outer diffusion, as well as chemical adsorption, which was the dominant mechanism. Based on the results of the thermodynamic experiment, the adsorption process was a nonspontaneous and exothermic reaction process. Furthermore, the presence of Cu2þ, Zn2þ, and Cr3þ ions significantly promoted the adsorption of OFL, but the presence of Cd2þ and Pb2þ ions inhibited its adsorption. At the same time, the presence of the ionic strength and fulvic acid remarkably restricted the adsorption process. These findings confirmed that electrostatic interactions, ion exchange, intermolecular hydrogen bonds, and halogen bond cooperation were the main adsorption mechanisms. This paper mainly discusses the interaction between combinations of pollutants with microplastics, which provides theoretical guidance for the interface behavior, migration and transformation of marine microplastics in the actual environment.
Keywords: Adsorption; Polyvinyl chloride Levofloxacin; Microplastic; Mechanism