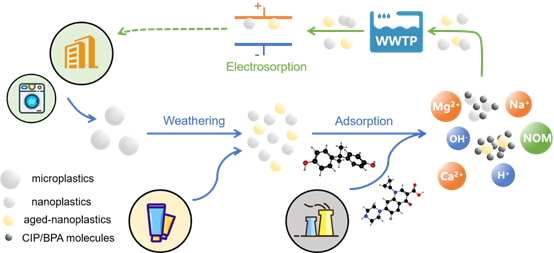
Abstract: Micro/nanoplastics have raised worldwide concern with extensive research on its transfer, toxicity and removal. However, the primary environmental process-adsorption of nanoplastics has not been uncovered since the discovery of nanosized plastics. Here, we synthesized nanoscale polystyrene (PS) particles with mean diameter of ~40 nm to avoid unknown properties from purchased ones, and thoroughly investigated its adsorption towards two typical pharmaceuticals and personal care products (PPCPs) with distinct characteristics, which are antibiotic (ciprofloxacin) and endocrine disruptor (bisphenol-A). Moreover, UV radiation is applied to simulate aging process in natural cases, and the carbonyl index derived from FTIR spectra increased clearly from 0.183 to 0.387. The adsorption capacity at equilibrium of CIP and BPA increased from 0.15 to 4.07 to 4.92 and 8.71 mg/g after weathering, respectively. Besides, the effect of environmental factors (pH, humic acid, salinity and cations) was also studied. Furthermore, electrosorption technology is applied to remove nanoplastics in solution for the first time, with the
capacity of 0.707 g nano-polystyrene/g AC and 0.322 g aged-nano-polystyrene/g AC, suggesting that adsorption under electric field is presumably a feasible tertiary treatment method targeted at nanoplastics in wastewater treatment plants (WWTPs).
Keywords: Microplastics; Nanoplastics; Interfacial interaction; Removal; Electrosorption