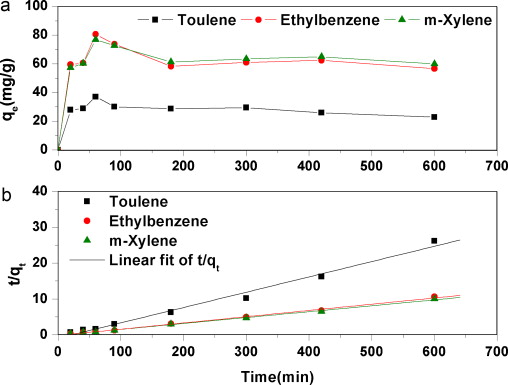
ABSTRACT: The purified and oxidized multi-walled carbon nanotubes (MWCNTs) with different oxygen contents are employed as adsorbents to study their physicochemical properties and adsorption behaviors of toluene, ethylbenzene and m-xylene (TEX) in aqueous solutions. The results demonstrate that adsorption capacity is significantly enhanced for 3.2% surface oxygen, but is dramatically reduced for 5.9% oxygen concentration. The adsorption kinetics is investigated and fitted with pseudo-second-order model. The adsorption isotherms are found to be fitted with Langmuir model. More interestingly, with the increasing of surface oxygen content, maximum adsorption capacities firstly increased, and then, began to decrease. In the first stage, dispersion is the most important factor. A better dispersive interaction increases the available adsorption sites, which consequently can be favorable for the aqueous phase adsorption. Therefore, maximum adsorption capacity is remarkably enhanced with the increasing of oxygen content, which is according with our results. However, in the second stage, when oxygen content increases to a certain extent, hydroxyl groups cause water clusters formation on the surface or tube end of MWCNTs, which hinder the interaction between TEX and MWCNTs. Consequently, more oxygen content leads to the decrease in maximum adsorption capacity. The decrease indicates that the formation of water clusters plays a more important role than the better dispersion of MWCNTs for TEX adsorption.