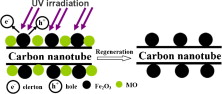
ABSTRACT: We report a simple and easy method to fabricate magnetic carbon nanotubes (CNTs) by Fenton’s reagent method without the addition of any cations. H2O2 was added slowly into the FeSO4 solution mixed with purified CNTs, and the resulting reactants were placed into a quartz tube to undergo heat treatment under a nitrogen/hydrogen flow. Iron oxide (Fe2O3) nanoparticles were uniformly dispersed on CNTs without any pretreatment such as strong acid or covalent functionalization processes. The as-produced magnetic CNTs were used
as an adsorbent for
removal
of methyl orange (MO) dye from
aqueous solutions. Adsorption experiments indicated that the magnetic CNTs have good adsorption capacity (qe) of MO (28
mg/g). The Freundlich isotherm model fitted the experiment data
better than the Langmuir
isotherm
mode. The mean energy of adsorption was calculated as 3.72
kJ/mol based on the Dubinin–Radushkevich model, which suggests that the
removal process was dominated by physical adsorption. Kinetic regression results showed that the adsorption kinetics was more accurately represented by a pseudo second-order model. Intra-particle diffusion
was
involved in the adsorption process, but it was not the only rate-controlling step. More importantly, a new photocatalytic regeneration technology can be enabled by the high nanoscale iron oxide loading (50%). The magnetic CNT adsorbents could be effectively and quickly separated by applying an external magnetic field and regenerated by UV photocatalysis. Therefore, CNTs/λ-Fe2O3 hybrid is a promising magnetic nanomaterial for preconcentration and separation of organic pollutants for environmental remediation.