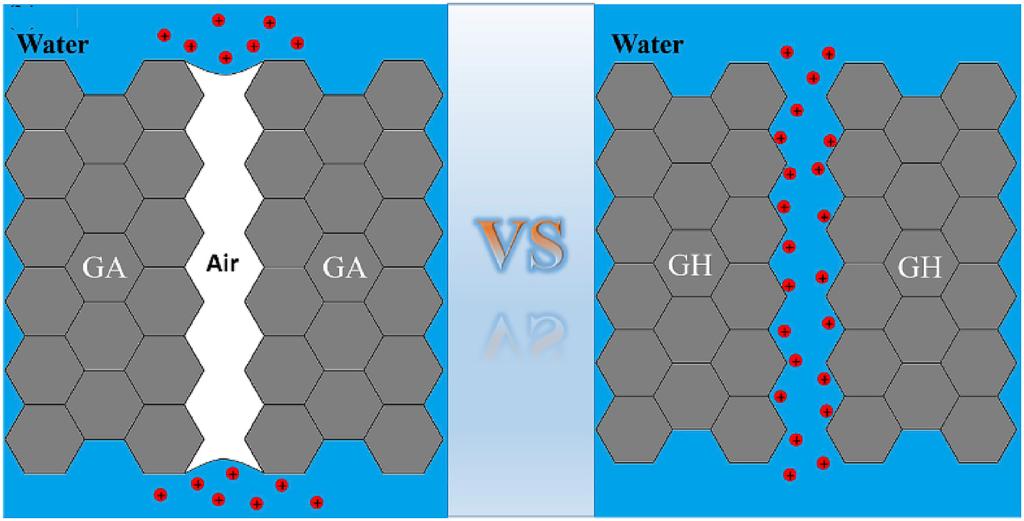
ABSTRACT: Graphene gel is an excellent material for capacitive deionization (CDI) because of its high specific surface area, three-dimensional structure and binder-free preparation. Current studies have been primarily focused on the design and synthesis of new graphene gel materials. However, there have been no studies comparing the desalination performance of graphene hydrogel (GH) and graphene aerogel (GA), the two primary CDI materials. In this work, GH and GA were synthesized and applied in CDI. A range of characterization methods were used to analyze the microstructure of the GA and GH electrode materials. The results showed that the electrosorption capacities of GH and GA were 49.34 and 45.88 mg g−1, respectively, in sodium chloride solution with an initial concentration of 500 mg L−1 at 2.0 V. A water-enhanced function mechanism was proposed to explain the differences in desalination performance.