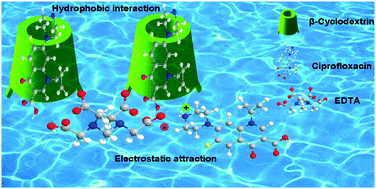
ABSTRACT: β-Cyclodextrin (β-CD) was cross-linked with ethylene diaminetetraacetic acid (EDTA) to prepare ethylene diaminetetraacetic acid–β-cyclodextrin (EDTA/β-CD). EDTA/β-CD was applied for the removal of ciprofloxacin (CPX) from water. The qualitative analysis of this material was performed using Fourier transform infrared (FT-IR) spectroscopy. Kinetic experimental data showed that the adsorption of CPX by EDTA/β-CD reached equilibrium state quickly, and corresponded to the pseudo-second-order model. Non-linear fitting parameters of the adsorption isotherm studies showed that the adsorption process matched the Dubinin–Radushkevich (D–R) isotherm model. Because of the electrostatic attraction between the adsorbent and adsorbate, the competitiveness of the adsorbate and the ions in the solution, and the salting-out effect, the adsorption process will be affected by the pH and ionic strength. EDTA/β-CD has low toxicity, is environmentally friendly, cheap, and has an excellent adsorption performance, so it is an excellent material for the removal of organic pollutants from wate