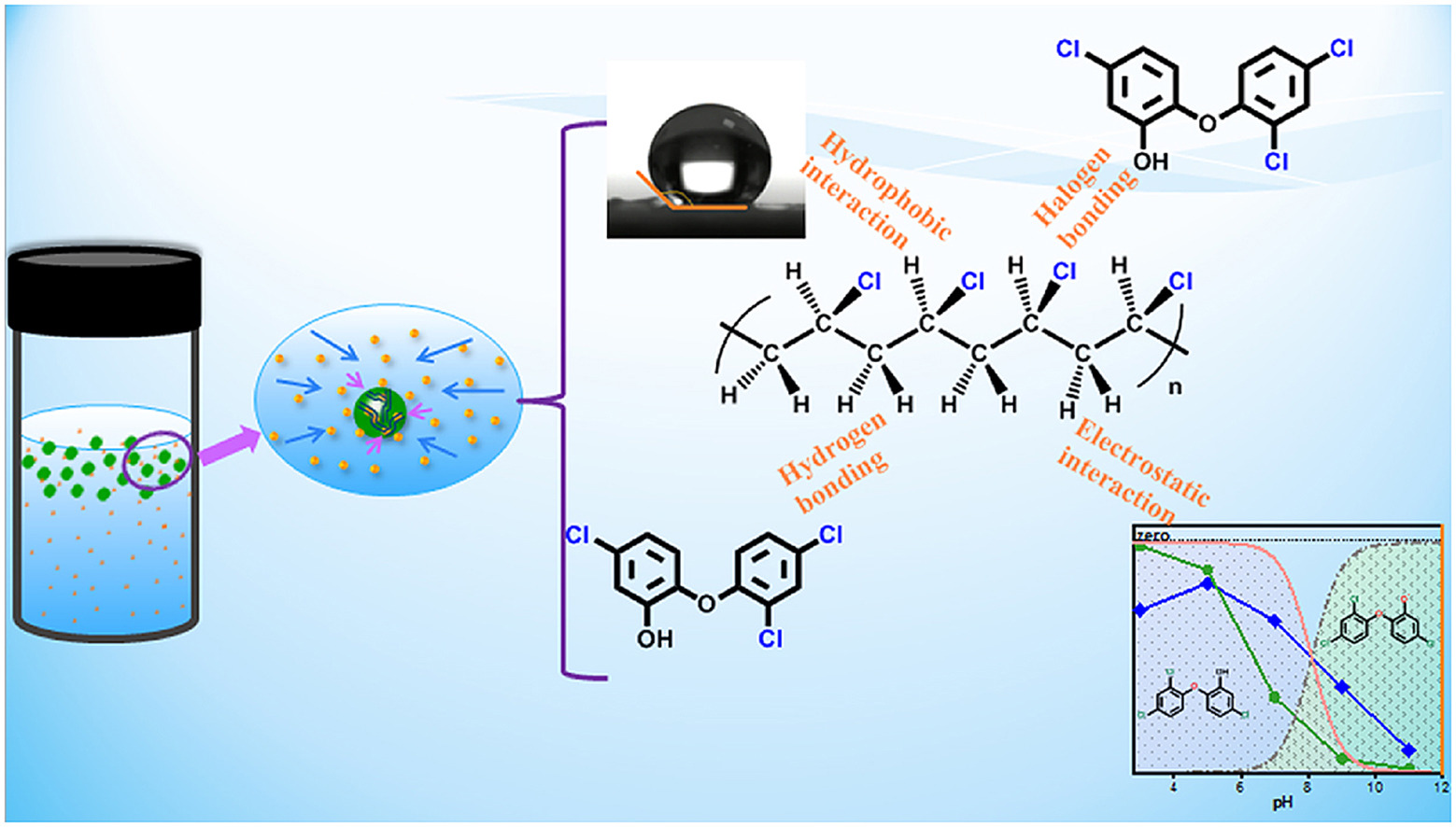
Abstract
Microplastics in water environment and its ability to load various environmental pollutants have attracted wide attention in recent years. However, effect of microplastic size on the adsorption behavior of environmental pollutants and interaction mechanism has not been thoroughly explored. In this study, triclosan (TCS) was selected as model pollutant, and polyvinyl chloride (PVC) with different particle sizes (small size (<1 μm) is recorded as PVC-S and PVC-L means large particle size of about 74 μm) were used as the typical microplastics, the adsorption behavior of TCS on PVC was investigated by studying kinetics, isotherms, and other influencing factors, such as pH and salinity. The results indicate PVC-S has greater distribution coefficient kdvalues of TCS (1.35 L/g > 1.05 L/g) and stronger adsorption capacity (12.7 mg/g > 8.98 mg/g) compared with PVC-L, which may be due to higher specific surface area, stronger hydrophobicity and relatively small electronegative property of PVC-S. Moreover, the initial pH value and salinity of the solution played crucial role in the adsorption process. The distribution diffusion mechanisms (including liquid-film diffusion and intra-particle diffusion), hydrophobic interaction, electrostatic interaction, halogen bonding, and hydrogen bonding may be the important reasons for adsorption. These findings show that MPs with different particle sizes have vary adsorption behaviors and load capacities for environmental pollutants, which deserve our further concerned.
 |
| Graphical abstract |