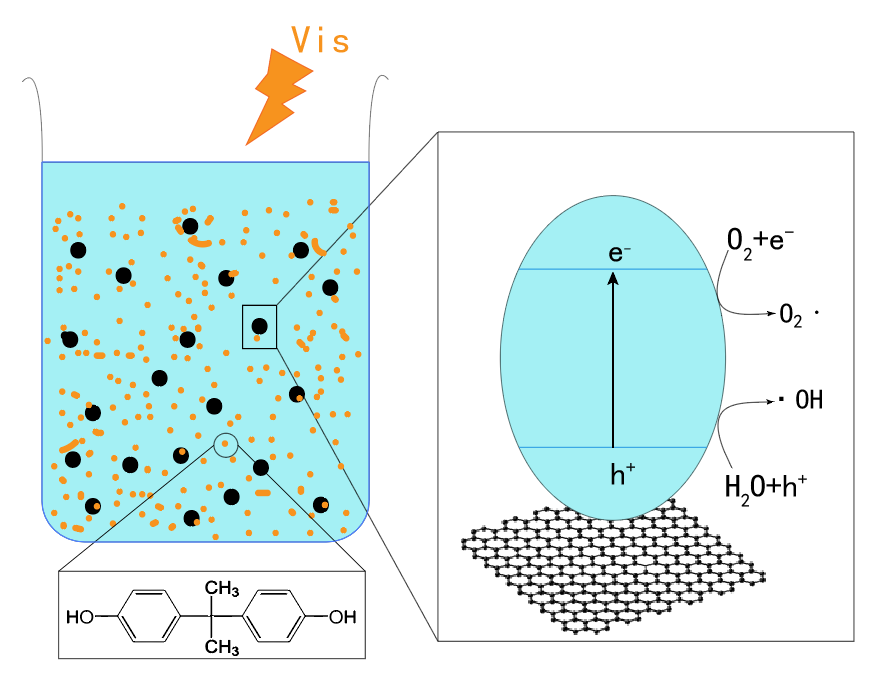
Reduced graphene oxide–titanium dioxide photocatalyst (rGO–TiO
2) was successfully synthesized by the hydrothermal method. The rGO–TiO
2
was used as photocatalyst for the degradation of bisphenol A (BPA), which is a typical endocrine disruptor of the environment. Characterization of photocatalysts and photocatalytic experiments under different conditions were performed for studying the structure and properties of photocatalysts. The characterization results showed that part of the anatase type TiO
2
was converted into rutile type TiO
2
after hydrothermal treatment and 1% rGO–P25 had the largest specific surface area (52.174 m
2/g). Photocatalytic experiments indicated that 1% rGO–P25 had the best catalytic effect, and the most suitable concentration was 0.5 g/L. When the solution pH was 5.98, the catalyst was the most active. Under visible light, the three photocatalytic mechanisms were ranked as follows: O
2
•−
> •OH > h
+. 1% rGO–P25 also had strong photocatalytic activity in the photocatalytic degradation of BPA under sunlight irradiation. 1% rGO–P25 with 0.5 g/L may be a very promising photocatalyst with a variety of light sources, especially under sunlight for practical applications.