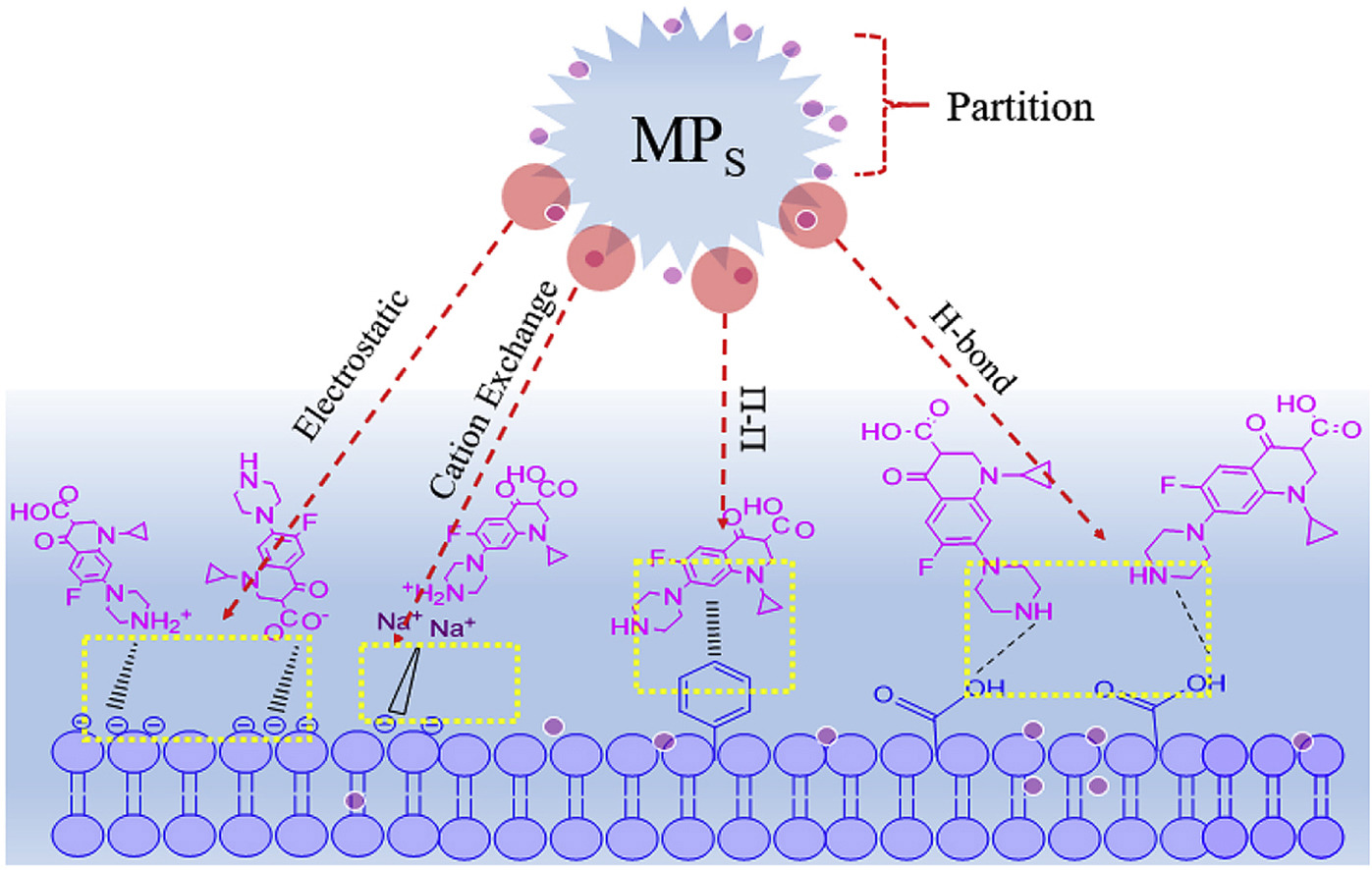
ABSTRACT: Virgin microplastics undergo aging and form oxygen-containing functional groups when they enter the environment. Therefore, the sorption of organic pollutants onto microplastics is not limited to hydrophobic organic pollutants and can also occur with hydrophilic organic pollutants. Therefore, understanding the sorption behaviors and mechanism between aged microplastics and hydrophilic organic pollutants is essential for evaluating the real effects of microplastics in the environment. We investigated the impacts of the UV-accelerated aging of polystyrene (PS) and polyvinylchloride (PVC) on their sorption interactions with ciprofloxacin (CIP). The results of infrared spectroscopy (IR) and scanning electron microscopy (SEM) showed significant surface oxidation and localized microcracks on the aged microplastics. The sorption kinetics and isotherms models indicated that the sorption capacity of aged microplastics is higher than that of pristine microplastics, and their physical interactions, including partitioning, electrostatic interactions, and intermolecular hydrogen bonding, were the dominant mechanism, as demonstrated by FTIR analysis. Moreover, the sorption capacity of the pristine microplastics decreased as the degree of crystallinity increased, whereas the opposite trend was observed with aged microplastics, which means that the crystallinity is not the controlling factor. In addition, salinity suppressed adsorption on all the tested microplastics. The pH influences the electrostatic attraction between the microplastics and CIP because CIP has a different charge at different pH values. The results presented herein confirm the importance of studying the adsorption between hydrophilic organic pollutants and aged microplastics because ultimately, all microplastics become aged. Moreover, the effects of aged microplastics with adsorbed hydrophilic chemicals on organisms need to be further studied.