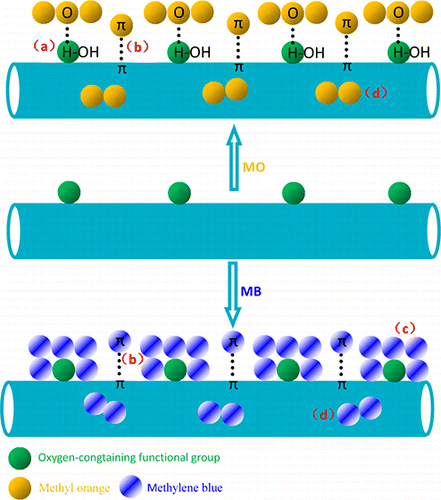
ABSTRACT: An alkali-acitvated method was explored to synthesize activated carbon nanotubes (CNTs-A) with a high specific surface area (SSA), and a large number of mesopores. The resulting CNTs-A were used as an adsorbent material for removal of anionic and cationic dyes in aqueous solutions. Experimental results indicated that CNTs-A have excellent adsorption capacity for methyl orange (149 mg/g) and methylene blue (399 mg/g). Alkali-activation treatment of CNTs increased the SSA and pore volume (PV), and introduced oxygen-containing functional groups on the surface of CNTs-A, which would be beneficial to improving the adsorption affinity of CNTs-A for removal of dyes. Kinetic regression results shown that the adsorption kinetic was more accurately represented by a pseudo second-order model. The overall adsorption process was jointly controlled by external mass transfer and intra-particle diffusion, and intra-particle diffusion played a dominant role. Freundlich isotherm model showed a better fit with adsorption data than Langmuir isotherm model. Adsorption interactions of dyes onto CNTs-A from aqueous solutions were investigated using Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD), and Brunauer–Emmett–Teller (BET) method. The remarkable adsorption capacity of dye onto CNTs-A can be attributed to the multiple adsorption interaction mechanisms (hydrogen bonding, π–π electron–donor–acceptor interactions, electrostatic interactions, mesopore filling) on the CNTs-A. Results of this work are of great significance for environmental applications of activated CNTs as a promising adsorbent nanomaterial for organic pollutants from aqueous solutions.