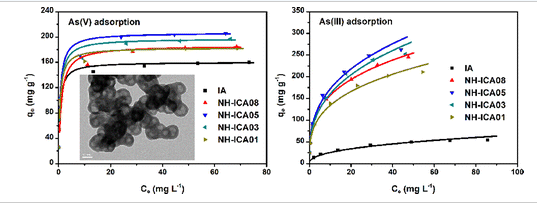
ABSTRACT:Recently, metal oxides with novel nanostructured architectures have been prepared by annealing the polyol-based metal alkoxides for water treatment. However, these materials often exhibit relatively low adsorption capacities possibly attributable to the decomposition of surface groups during the calcination process. In this work, we successfully synthesized a novel nanostructured hollow iron−cerium alkoxide (NH-ICA) with a high surface area and abundant surface functional groups through an ethylene glycol mediated solvothermal method. Cerium ion doping significantly influenced the morphologies, microstructures and adsorption performance of NH-ICAs. Interestingly, the synthesized NH-ICAs showed significantly higher affinity to As(III) than the iron alkoxide material without cerium doping. Moreover, a much higher adsorption capacity of the NH-ICAs for As(III) than As(V) was found. When the molar ratio of Fe to Ce was 5:1, the product with uniform nanostructured hollow architectures exhibited the best adsorption capacities for both As(V) and As(III) (206.6 and 266.0 mg g−1, respectively). The mechanistic study revealed that As(V) adsorption involved ion-exchange between the As(V) species and three types of negatively charged groups, including surface hydroxyl groups, CO32− and unidentate carbonate-like species. For As(III) adsorption, surface complexing was proposed. A broad adaptation pH range for both As(V) and As(III) adsorbed by the resulting product indicates its promising application perspective for decontamination of arsenic-polluted water.