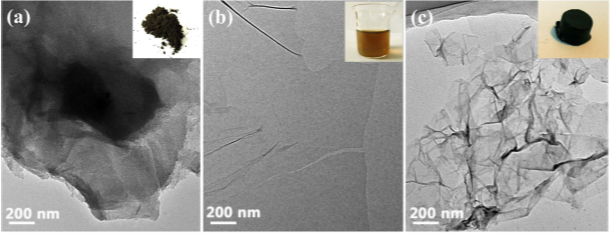
ABSTRACT: An environmentally benign and efcient hydrothermal reduction method was applied for the preparation of three-dimensional (3D) porous graphene hydrogel (GH) adsorbents. The physicochemical properties of GH granules were systematically characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD), Raman spectra and Brunauer-Emmett-Teller (BET) method. GH granules showed an excellent adsorption capacity (235.6 mg/g) for ciprofloxacin via combined adsorption interaction mechanisms (e.g. π-π EDA interaction, hydrogen bonding, and hydrophobic interaction). Moreover, reducing the size of the hydrogels can signifcantly accelerate the adsorption process and enhance the removal efciency of pollutants from aqueous solution. Water (more than 99 wt%) within hydrogels played a key role in enhancing adsorption performance. The GO hydrogels exhibited an excellent adaptability to environmental factors. These fndings demonstrate that GH granules are promising adsorbents for the removal of antibiotic pollutants from aqueous solutions.