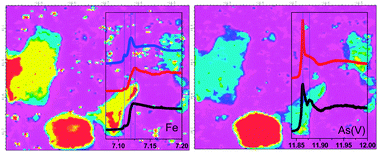
ABSTRACT:Magnetic iron oxide/graphene oxide (MGO) with high iron loading (51 wt%) has been successfully synthesized using the co-precipitation method, and then used as adsorbents for the removal of arsenate and arsenite from aqueous solutions. The resulting MGO possesses desirable magnetic properties (12.8 emu g��1) and excellent adsorption properties for the removal of As(III)and As(IV) with significantly enhanced adsorption capacities of 54.18 mg g��1 and 26.76 mg g��1, respectively. These values are much higher than those of other GO-based composites reported previously. The kinetic, equilibrium and environmental effects (pH, ionic strength, coexist anion) of MGO were obtained experimentally. A synchrotron-based X-ray fluorescent microprobe was used to generate elemental distribution maps of adsorbents; the results suggest that As(V) became preferentially associated with iron oxides during the adsorption process, and that the distribution of Fe is directly correlated with the distribution of As.