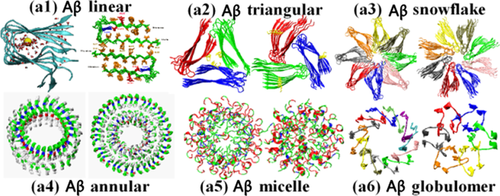
ABSTRACT:The misfolding and aggregation of proteins and peptides into amyloid fibrils are believed to be responsible for the dysfunction and death of neuron cells in many neurodegenerative diseases. Resolving the atomic structures of amyloid peptides at different aggregation stages by molecular simulations has opened new ways to probe the molecular mechanisms of amyloid aggregation, toxicity, and inhibition, as well as to validate computational data with available experimental ones. In this review article, we summarize some recent and important findings on: 1) a number of atomic structures of amyloid oligomers with typical β‐sheet‐rich conformations, related to amyloid aggregation; 2) different amyloid peptide‐induced membrane‐disruption mechanisms, related to amyloid toxicity; and 3) rational design of different amyloid inhibitors capable of preventing amyloid aggregation and toxicity, related to amyloid inhibition. All these findings will provide some mechanistic implications for molecular mechanisms of amyloid aggregation, toxicity, and inhibition, which are fundamentally and practically important for the treatment of amyloid diseases.