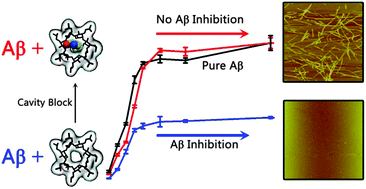
ABSTRACT: Amyloid deposits of misfolded amyloid-b protein (Ab) on neuronal cells are a pathological hallmark of Alzheimer’s disease (AD). Prevention of the abnormal Ab aggregation has been considered as a promising therapeutic strategy for AD treatment. To prevent reinventing the wheel, we proposed to search the existing drug database for other diseases to identify potential Ab inhibitors. Herein, we reported the inhibitory activity of HP-b-cyclodextrin (HP-b-CD), a well-known sugar used in drug delivery, genetic vector, environmental protection and treatment of Niemann–Pick disease type C1 (NPC1), against Ab1–42 aggregation and Ab-induced toxicity, with the aim of adding a new function as a sugar-based Ab inhibitor. Experimental data showed that HP-b-CD molecules were not only nontoxic to cells, but also greatly inhibited Ab fibrillization and reduced Ab-induced toxicity in a concentrationdependent manner. At an optimal molar ratio of Ab : HP-b-CD = 1 : 2, HP-b-CD enabled the reduction of 60% of Ab fibrils and increased the cell viability to 92%. Such concentration-dependent inhibitor capacity of HP-b-CD was likely attributed to several combined effects, including the enhancement of Ab–HP-bCD interactions, prevention of structural transition of Ab peptides towards b-sheet structures, and reduction of self-aggregation of HP-b-CD. In parallel, molecular simulations further revealed the atomic details of HP-b-CD interacting with the Ab oligomer, showing that HP-b-CD had a high tendency to interact with hydrophobic residues of Ab in two b-strands and the N-terminal tail. More importantly, we identified that the inner hydrophobic cavity of HP-b-CD was a key active site for Ab inhibition. Once the inner cavity of HP-b-CD was blocked by a small hydrophobic molecule of ferulic acid, HP-b-CD completely lost its inhibition capacity against Ab. Given the already established pharmaceutical functions of HP-b-CD in drug delivery, our findings suggest that HP-b-CD has great potential to be designed as a sugar-based Ab inhibitor.