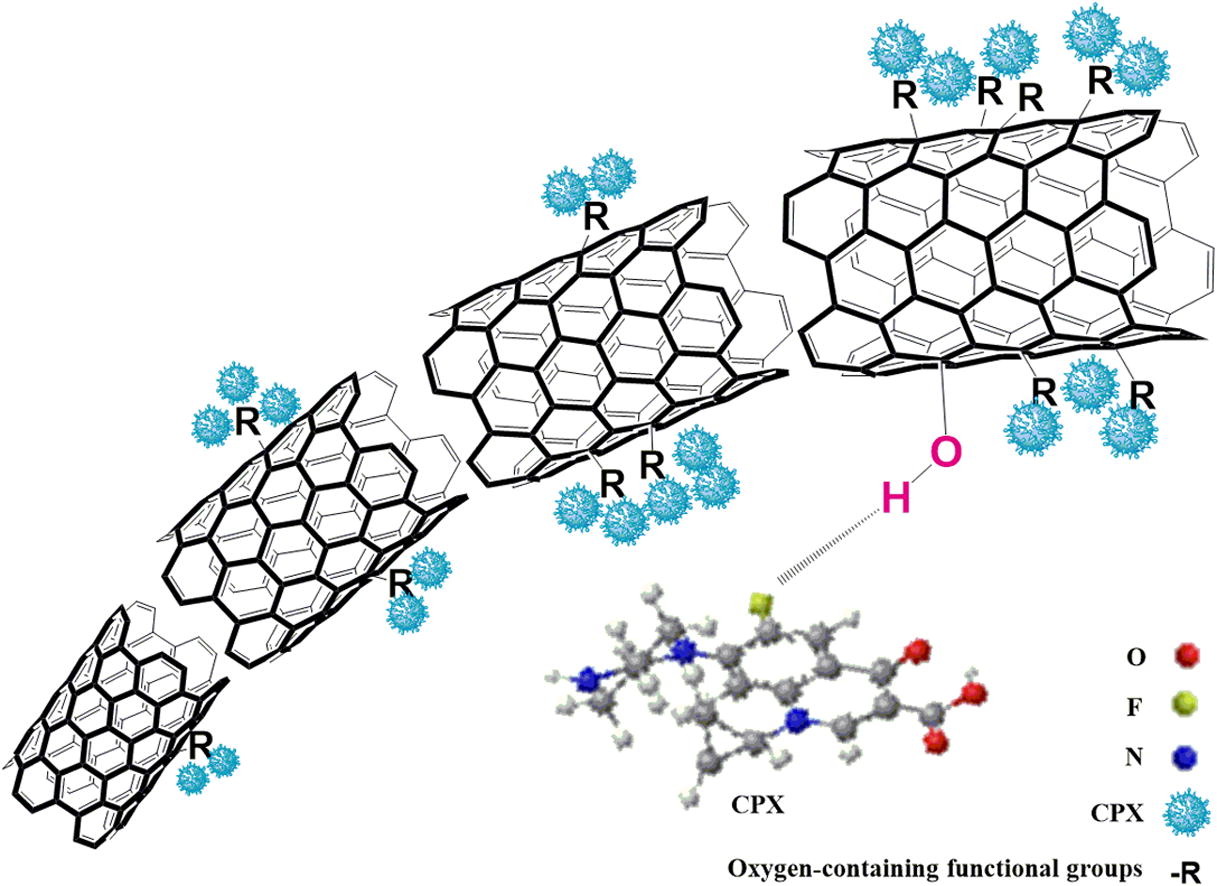
ABSTRACT: The oxidized multi-walled carbon nanotubes (MCNTs) were used as adsorbents to investigate the effects of oxygen contents on adsorption properties of ciprofloxacin (CPX). With the oxygen content increasing from 2.0% to 5.9%, the normalized maximum adsorption capacity of CPX appeared growing state. However, the increment rate became slower, which is mainly attributed to p–p electron donor–acceptor interaction. The promotion of hydrophilicity and dispersibility, and the inhibition of water cluster had played a coordinate role in the whole adsorption process of CPX onto MCNTs. The isotherm adsorption data were more appropriate to Dubinin–Radushkevich and Langmuir model. Moreover, the MCNTs with the best adsorption properties was chosen to investigate adsorption kinetics and the effect of environmental factors (dosage, and pH, ionic strength) on CPX adsorption. The experimental kinetic data showed that intra-particle diffusion and outer diffusion may both present in the removal process to control the adsorption rate. CPX adsorption strongly depended on the pH of the solution. The alkaline condition was not conducive to the adsorption of CPX on MCNTs. However, the ionic strength had no significant effect on the adsorption capacity of CPX onto MCNTs. Therefore, the electrostatic interaction may be the main adsorption mechanism in the adsorption process.