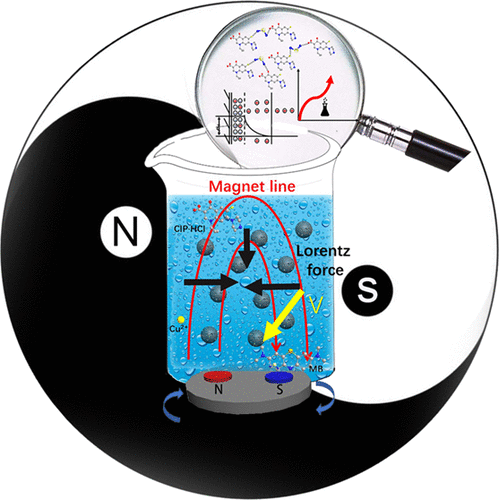
ABSTRACT:Novel, efficient bioadsorbent sodium alginate/graphene/l-cysteine (SA/GR/l-Cys) beads were prepared and used for magnetic field (MF)-assisted adsorption of pollutants. SA/GR/l-Cys has excellent mechanical properties, with a breaking stress of 3.5 MPa at 79.8%, an elastic modulus of 5.0 MPa, low swelling properties (average swelling ratio 300%), and good adsorption properties toward organic pollutants and heavy metal ions. A rotating magnetic field (RMF) was shown to have a better influence than a static magnetic field (SMF) on adsorption, with enhanced adsorption capacities 5-fold greater than those of the SMF. We investigated the different adsorption mechanisms of model contaminants through Fourier transform infrared spectroscopy, potential, and X-ray photoelectrons spectroscopy. Formation of new hydrogen bonds, change in potential, and acceleration in chemical reactions strongly influenced the adsorption process under the RMF. In fixed-bed column adsorption, the breakthrough time for column adsorption increased, and the adsorption capacity improved by 30.66%. The costs and practical applications of SA/GR/l-Cys under RMF were also analyzed. This work demonstrated that SA/GR/l-Cys could serve as a promising adsorbent for water pollutants under RMF exposure and could be used in practical applications.